About Davies Diagnostics
Since 1988 we have expanded by introducing new products and technology into the microbiology, haematology, immunology, serology, veterinary and general laboratory markets.
The Davies Diagnostics Mission
Davies Diagnostics regularly engages with pathology opinion leaders, advertises in relevant journals, and exhibits at meetings. We send technical and commercial mailings, ensuring our reputation for quality products and excellent customer service. We only select new products that meet our high standards and support them with full marketing and sales efforts.
Our Certifications
We have a team of very enthusiastic people who are working hard to grow the Company. In testimony to this enthusiastic spirit, we proudly achieved ISO 9001 certification in July 2001. Davies Diagnostics (Pty) Ltd was the first independent diagnostics distributor to achieve such status in South Africa! We are ISO 13485 Certified.
Who we are
Davies Diagnostics (Pty) Ltd was founded in 1983 primarily to distribute the range of products manufactured by Mast Laboratories, United Kingdom. Between 1983 -1988 we firmly established ourselves in the microbiology area.
Featured Products

AIX1000®
The AIX1000® is an efficient automated system that can process up to 192 samples in 90 minutes, offering full sample traceability with integrated barcode reading. It utilizes a proprietary image recognition algorithm for objective results, reducing labor and turnaround time. The system provides easy access to test results, improved database management, and remote data archiving capabilities. It.s compatible with the AIX1000® RPR Reagent kit and features an expandable status log for enhanced functionality.
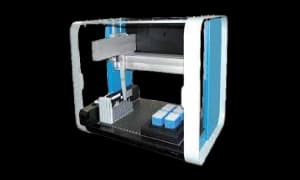
NEO Sample Management®
NEO Sample Management® offers a comprehensive solution for liquid sample management at -80°C. The system includes an automated liquid handling robot for secure sample transfer, NEO Plates with integrated 2D barcodes, and NEO Manager software for efficient tracking and retrieval. This product range ensures traceability and security for various laboratory settings, including clinical, serum banks, blood banks, biotechnology, and diagnostics. NEO Sample Management is globally available, catering to diverse research and testing environments.

Virclia® Lotus
VirClia® Lotus is a flexible, continuous-loading diagnostic system that allows random access for samples and reagents, even during operation. It features a STAT function for prioritizing urgent tests and offers total flexibility as a monotest solution. This system can handle both routine and low-volume infectious serology tests, consolidating parameters typically performed with ELISA, IFA, or Rapid Tests into a single CLIA platform, streamlining laboratory workflows.
Our Major Clients
Our Services
We deliver innovative diagnostic supplies and services, suited to your needs. Enjoy expert technical support and engaging webinars to drive exceptional performance in your laboratory and clinical practice.
Diagnostic Product Supply
Since 1988 we have expanded by introducing new products and technology into the microbiology, haematology, immunology, serology, veterinary and general laboratory markets.
